Plain Language Summary of a Systematic Review
Key messages:
- We found data from human studies only on two TAAR1 agonists. These medications made little to no difference in improving symptoms of psychosis compared to sugar pills (placebo) but seemed to be associated with fewer adverse events compared to current antipsychotics in people with psychosis when taken between 4-6 weeks.
- In mice, we studied increased movement that is used to mimic psychosis in animals and showed that TAAR1 agonists had some positive effects.
- There are other TAAR1 agonists being developed, but they have not been studied in humans yet. More evidence is needed on these additional TAAR1 agonists and how they may improve symptoms of psychosis.
What is psychosis?
Psychosis is a collection of mental health symptoms that distort reality and is commonly experienced in people who have schizophrenia. Psychosis can be extremely distressing and reduce a person's ability to carry out day-to-day tasks. Symptoms of psychosis include a person seeing and hearing things which are not there, such as voices (hallucinations), a person having false beliefs which are not easy to change or shared by others (delusions) or having disordered thinking and speaking.
How is psychosis currently treated?
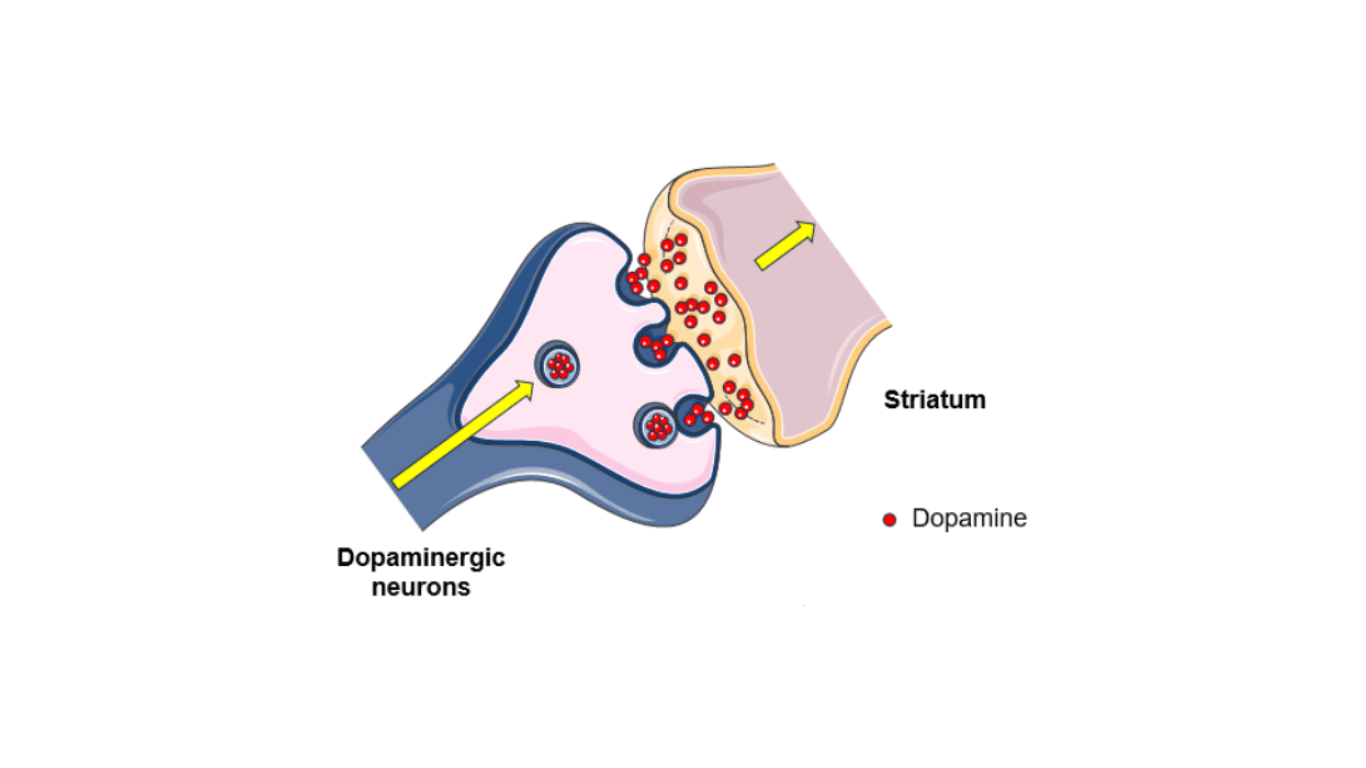
Certain chemical messengers in the brain, such as dopamine, are believed to be associated with psychosis. It is thought that if we can change the levels of dopamine, we can improve symptoms of psychosis. Current antipsychotic medications target brain receptors which respond to dopamine, however, these drugs do not work for all people and have uncomfortable side effects, especially when taken for a long time. Thus, it is important to discover better treatments for psychosis, such as TAAR1 agonists.
What are TAAR1 agonists?
Trace Amine-Associated Receptor 1 (TAAR1) agonists are a new class of drug which target different receptors in the brain, namely the receptor of trace amines, a neuromodulator. This receptor somehow affects how dopamine is made and produced in the brain. We currently do not know how this receptor works exactly. However, researchers think that its mechanism is what distinguishes TAAR1 agonists from other drugs for psychosis and it can explain why TAAR1 agonists may be better in terms of adverse events.
What did we want to find out?
We analysed the best available data on TAAR1 agonists to understand:
- Do TAAR1 agonists reduce symptoms of psychosis?
- What are the adverse events (such as movement disorders, weight gain, and vomiting ) caused by TAAR1 agonists?
- What are the mechanisms behind TAAR1 agonists? Can we explain how these drugs work?
The Figure was partly generated using Servier Medical Art licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
What did we do?
We searched available evidence investigating the effects of TAAR1 agonists in humans and animals.
Although it is not possible to study the same symptoms of psychosis in animals, we need animal models to understand how the drugs work and the effect of the medication on the brain. One way of mimicking psychosis in animals is by giving them a drug which increases dopamine, and, as an effect, the animal moves around more (this is called “increased locomotion”). If a drug reverses these behaviours, it indicates it could be a good candidate for improving symptoms of psychosis.
This review is part of a living systematic review. This means that it will be updated continuously, as new evidence emerges.
What did we find?
Human Evidence:
- For human studies, we found 20 studies, 9 of which had usable data from a total of 1,683 adult participants of both genders. Of these 9 studies: 4 studies investigated the effects of taking TAAR1 agonist as either a single dose or for under 2 weeks, and 5 studies investigated effects between 4-6 weeks. Studies focused on a range of conditions, including healthy participants, and 5/9 studies investigated people with schizophrenia. TAAR1 agonists were compared to either a placebo control or a currently approved antipsychotic.
- There was usable data from only two TAAR1 agonists.
- These TAAR1 agonists showed they may make little to no difference in symptoms of psychosis. - These TAAR1 agonists are associated with fewer adverse events being reported than current antipsychotic drugs, when taken between 4-6 weeks. They had more adverse events than a sugar pill (placebo) when taken as a single dose.
- There was no usable data on the mechanisms behind TAAR1 agonists so we cannot draw conclusions about how TAAR1 agonists work.
Animal Evidence:
- Fifteen animal studies were included in the review, with each study containing multiple experiments, resulting in data from more than 120 experiments. All studies investigated the effect of a TAAR1 agonist on an animal model of psychosis compared to control conditions similar to placebo in human studies or other antipsychotics.
- There was available data from ten different TAAR1 agonists, including ulotaront but not ralmitaront.
- TAAR1 agonists cause a large reduction in locomotive behaviour in animal models of psychosis, but the positive effects were potentially smaller than already licensed antipsychotics.
- The efficacy of TAAR1 agonists may depend on the dose of the TAAR1 agonist.
- There was no usable data on adverse events.
- There was no usable animal data on the mechanisms of TAAR1 agonists.
What are the limitations of the evidence?
We are not very confident in the conclusions we can draw from human evidence, however, have moderate confidence in the evidence from animal studies. For both animal and human studies, the studies were of small sample sizes, short durations, and had inconsistent findings, leading to mixed results. For human studies, there was no data from children, and so the current conclusions may be limited to adult populations. There was limited data on adverse events, and this was inconsistently reported across studies.
Results between the animal studies and human studies do not align, but both suggested that current TAAR1 agonists may be less effective than already licensed antipsychotic. We found no usable data for how TAAR1 agonists work. Importantly, the human data is only drawn from two TAAR1 agonists, and other TAAR1 agonists may show different efficacy and impact on adverse events. Overall, more evidence is needed on these additional TAAR1 agonists and their mechanisms, to understand how they may improve symptoms of psychosis.
How up to date is this evidence?
The first and current iteration of the review was conducted in November 2023.
You can find the full Systematic Review here: https://wellcomeopenresearch.org/articles/9-182/v1
The full data can be downloaded here:

